Hot Air Balloons Gas Laws . Why does air rise when it is heated?. The ideal gas law states that \[p v=n k t, \nonumber\] where \(p\) is the absolute pressure of a gas, \(v\) is the volume it. Gas laws many of our gas laws were discovered by balloonists. The relationship between temperature and volume: The operation of a hot air balloon depends on charles' law, the ideal gas law, and archimedes' principle. Have you ever wondered how hot air balloons work? The operator first ignites a propane burner to fill the balloon with. Just to lift an adult man's weight, you'd need a balloon about 4m (13ft) in radius with the air inside heated to a temperature of about 120°c (250°f). So we could say that charles' law describes how hot air balloons get light enough to lift off, and why a temperature inversion prevents convection currents in the atmosphere, and.
from stock.adobe.com
Gas laws many of our gas laws were discovered by balloonists. The relationship between temperature and volume: The operator first ignites a propane burner to fill the balloon with. So we could say that charles' law describes how hot air balloons get light enough to lift off, and why a temperature inversion prevents convection currents in the atmosphere, and. The ideal gas law states that \[p v=n k t, \nonumber\] where \(p\) is the absolute pressure of a gas, \(v\) is the volume it. Just to lift an adult man's weight, you'd need a balloon about 4m (13ft) in radius with the air inside heated to a temperature of about 120°c (250°f). Why does air rise when it is heated?. The operation of a hot air balloon depends on charles' law, the ideal gas law, and archimedes' principle. Have you ever wondered how hot air balloons work?
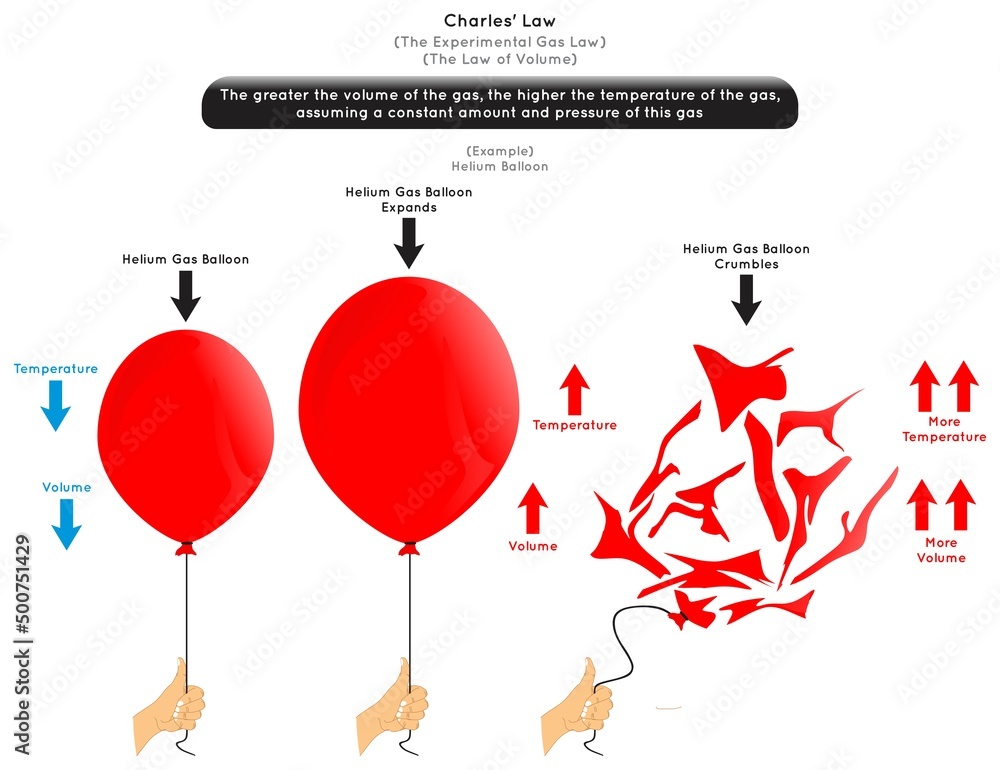
Vetor de Charles Law Infographic Diagram Example helium balloon when
Hot Air Balloons Gas Laws The operation of a hot air balloon depends on charles' law, the ideal gas law, and archimedes' principle. The ideal gas law states that \[p v=n k t, \nonumber\] where \(p\) is the absolute pressure of a gas, \(v\) is the volume it. So we could say that charles' law describes how hot air balloons get light enough to lift off, and why a temperature inversion prevents convection currents in the atmosphere, and. Gas laws many of our gas laws were discovered by balloonists. The operator first ignites a propane burner to fill the balloon with. Have you ever wondered how hot air balloons work? The relationship between temperature and volume: The operation of a hot air balloon depends on charles' law, the ideal gas law, and archimedes' principle. Just to lift an adult man's weight, you'd need a balloon about 4m (13ft) in radius with the air inside heated to a temperature of about 120°c (250°f). Why does air rise when it is heated?.
From www.thecoldwire.com
What Type Of Gas Is Used In Hot Air Balloons? (Everything To Know) Hot Air Balloons Gas Laws Have you ever wondered how hot air balloons work? The ideal gas law states that \[p v=n k t, \nonumber\] where \(p\) is the absolute pressure of a gas, \(v\) is the volume it. The relationship between temperature and volume: The operator first ignites a propane burner to fill the balloon with. Gas laws many of our gas laws were. Hot Air Balloons Gas Laws.
From mammothmemory.net
Newton's third law Examples Hot Air Balloons Gas Laws Just to lift an adult man's weight, you'd need a balloon about 4m (13ft) in radius with the air inside heated to a temperature of about 120°c (250°f). The operator first ignites a propane burner to fill the balloon with. So we could say that charles' law describes how hot air balloons get light enough to lift off, and why. Hot Air Balloons Gas Laws.
From www.youtube.com
Hot Air Balloon Ideal Gas Law Problem YouTube Hot Air Balloons Gas Laws Gas laws many of our gas laws were discovered by balloonists. The operation of a hot air balloon depends on charles' law, the ideal gas law, and archimedes' principle. The operator first ignites a propane burner to fill the balloon with. Why does air rise when it is heated?. The ideal gas law states that \[p v=n k t, \nonumber\]. Hot Air Balloons Gas Laws.
From www.ck12.org
The Behavior of Gases CK12 Foundation Hot Air Balloons Gas Laws The ideal gas law states that \[p v=n k t, \nonumber\] where \(p\) is the absolute pressure of a gas, \(v\) is the volume it. Why does air rise when it is heated?. The operator first ignites a propane burner to fill the balloon with. The relationship between temperature and volume: So we could say that charles' law describes how. Hot Air Balloons Gas Laws.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Hot Air Balloons Gas Laws The ideal gas law states that \[p v=n k t, \nonumber\] where \(p\) is the absolute pressure of a gas, \(v\) is the volume it. So we could say that charles' law describes how hot air balloons get light enough to lift off, and why a temperature inversion prevents convection currents in the atmosphere, and. Just to lift an adult. Hot Air Balloons Gas Laws.
From cartoondealer.com
Physics Balloon And Gas Experiments Cartoon Vector CartoonDealer Hot Air Balloons Gas Laws The ideal gas law states that \[p v=n k t, \nonumber\] where \(p\) is the absolute pressure of a gas, \(v\) is the volume it. Have you ever wondered how hot air balloons work? So we could say that charles' law describes how hot air balloons get light enough to lift off, and why a temperature inversion prevents convection currents. Hot Air Balloons Gas Laws.
From seattleballooning.com
How Hot Air Balloons Work? (It's Amazing) Seattle Ballooning Hot Air Balloons Gas Laws Gas laws many of our gas laws were discovered by balloonists. The ideal gas law states that \[p v=n k t, \nonumber\] where \(p\) is the absolute pressure of a gas, \(v\) is the volume it. The relationship between temperature and volume: So we could say that charles' law describes how hot air balloons get light enough to lift off,. Hot Air Balloons Gas Laws.
From www.tec-science.com
How does a hot air balloon work Buoyancy in gases tecscience Hot Air Balloons Gas Laws So we could say that charles' law describes how hot air balloons get light enough to lift off, and why a temperature inversion prevents convection currents in the atmosphere, and. The relationship between temperature and volume: Have you ever wondered how hot air balloons work? The ideal gas law states that \[p v=n k t, \nonumber\] where \(p\) is the. Hot Air Balloons Gas Laws.
From www.sophia.org
Combined Gas Law Examples Tutorial Sophia Learning Hot Air Balloons Gas Laws The operation of a hot air balloon depends on charles' law, the ideal gas law, and archimedes' principle. The ideal gas law states that \[p v=n k t, \nonumber\] where \(p\) is the absolute pressure of a gas, \(v\) is the volume it. Gas laws many of our gas laws were discovered by balloonists. Why does air rise when it. Hot Air Balloons Gas Laws.
From www.atmo.arizona.edu
Lecture 6 Ideal gas law, rising and sinking air Hot Air Balloons Gas Laws The operation of a hot air balloon depends on charles' law, the ideal gas law, and archimedes' principle. The ideal gas law states that \[p v=n k t, \nonumber\] where \(p\) is the absolute pressure of a gas, \(v\) is the volume it. The relationship between temperature and volume: Just to lift an adult man's weight, you'd need a balloon. Hot Air Balloons Gas Laws.
From kinderbilder.download
Wie Funktioniert Ein Heißluftballon kinderbilder.download Hot Air Balloons Gas Laws The operator first ignites a propane burner to fill the balloon with. Just to lift an adult man's weight, you'd need a balloon about 4m (13ft) in radius with the air inside heated to a temperature of about 120°c (250°f). The relationship between temperature and volume: Why does air rise when it is heated?. Gas laws many of our gas. Hot Air Balloons Gas Laws.
From www.slideserve.com
PPT Charles’ Law PowerPoint Presentation, free download ID6862132 Hot Air Balloons Gas Laws Just to lift an adult man's weight, you'd need a balloon about 4m (13ft) in radius with the air inside heated to a temperature of about 120°c (250°f). The relationship between temperature and volume: The operator first ignites a propane burner to fill the balloon with. Have you ever wondered how hot air balloons work? Gas laws many of our. Hot Air Balloons Gas Laws.
From www.thecoldwire.com
What Type Of Gas Is Used In Hot Air Balloons? (Everything To Know) Hot Air Balloons Gas Laws The operator first ignites a propane burner to fill the balloon with. The relationship between temperature and volume: The operation of a hot air balloon depends on charles' law, the ideal gas law, and archimedes' principle. Just to lift an adult man's weight, you'd need a balloon about 4m (13ft) in radius with the air inside heated to a temperature. Hot Air Balloons Gas Laws.
From uxuoikncpy.blogspot.com
How Hot Air Balloons Work Gas Laws We can rearrange the ideal gas law Hot Air Balloons Gas Laws Just to lift an adult man's weight, you'd need a balloon about 4m (13ft) in radius with the air inside heated to a temperature of about 120°c (250°f). The operator first ignites a propane burner to fill the balloon with. The ideal gas law states that \[p v=n k t, \nonumber\] where \(p\) is the absolute pressure of a gas,. Hot Air Balloons Gas Laws.
From www.youtube.com
Charles's Law of Ideal Gases Balloon experiment YouTube Hot Air Balloons Gas Laws The ideal gas law states that \[p v=n k t, \nonumber\] where \(p\) is the absolute pressure of a gas, \(v\) is the volume it. Why does air rise when it is heated?. The relationship between temperature and volume: Have you ever wondered how hot air balloons work? Gas laws many of our gas laws were discovered by balloonists. The. Hot Air Balloons Gas Laws.
From outdoortroop.com
How Do Hot Air Balloons Work? (A helpful guide with pictures) Outdoor Hot Air Balloons Gas Laws Have you ever wondered how hot air balloons work? The operation of a hot air balloon depends on charles' law, the ideal gas law, and archimedes' principle. The ideal gas law states that \[p v=n k t, \nonumber\] where \(p\) is the absolute pressure of a gas, \(v\) is the volume it. The operator first ignites a propane burner to. Hot Air Balloons Gas Laws.
From www.slideserve.com
PPT Heat Transfer PowerPoint Presentation, free download ID1606939 Hot Air Balloons Gas Laws The operation of a hot air balloon depends on charles' law, the ideal gas law, and archimedes' principle. The ideal gas law states that \[p v=n k t, \nonumber\] where \(p\) is the absolute pressure of a gas, \(v\) is the volume it. So we could say that charles' law describes how hot air balloons get light enough to lift. Hot Air Balloons Gas Laws.
From www.storyboardthat.com
hot cold air balloon charles law Storyboard by 05918d98 Hot Air Balloons Gas Laws Just to lift an adult man's weight, you'd need a balloon about 4m (13ft) in radius with the air inside heated to a temperature of about 120°c (250°f). Why does air rise when it is heated?. So we could say that charles' law describes how hot air balloons get light enough to lift off, and why a temperature inversion prevents. Hot Air Balloons Gas Laws.